“The slow vaccination process and the fear of a lagged recovery will continue to cause a reduction in risk assets,” say analysts at Link Securities. That said, it is true that “sooner or later, vaccination will take effect and one must be positioned for the day after,” say the experts at Intermoney. Amidst these sentiments, the Covid-19 vaccination campaign is one month in since it started in the European Union. Figures are still far from the rates needed to achieve the immunisation objectives: 1) by March, to have 80% of people over 80 years and of health care workers and nursing homes vaccinated, and 2) by the end of the summer (Sept-21), to have 70% of the population immunised. According to the latest available data and if the current vaccination rates are maintained, no member country will reach the objectives.
In the specific case of Spain, there have been over 1,200,000 doses administered since December 27 and more than 88,000 people have received the full two-dose pattern. The research team at Renta4 flags that “it would be necessary to multiply by 5.6x the current rate of daily doses administered in order to meet the goal of having 70% of the Spanish population immunised.” However, considering that the Autonomous Communities have already administered about 90% of the doses received, the bottleneck is currently not so much in the primary care centers but in the deliveries from the pharmaceutical companies. In fact, the tension between the EU and Astrazaneca continues in Europe, with politicians and pharmaceutical companies blaming each other.
The Anglo-Swedish pharma announced that it could not meet the number of doses of the vaccine it had committed to the European Union by cutting deliveries by up to 60% in the first four months of the year. AstraZeneca’s explanations did not convince the European Commission, which then accused the company of selling the doses to the highest bidder. Today the EU could announce the tightening of the rules for exporting Covid -19 vaccines outside the EU. So pharmaceutical companies may be required to obtain prior authorisation with detailed information about volumes and destination. Neither does the EU rule out the possibility of taking control of the vaccines’ production if these measures do not have the desired effect.
Today, in principle, European regulators are expected to recommend authorisation for emergency use in the region of the vaccine developed by AstraZeneca and the University of Oxford. If approved, it would be the third Covid-19 vaccine available in Europe after those from Pfizer and Moderna were approved on Dec. 21 and Jan. 6, respectively. However, a decision was made known yesterday from the German health authorities that they are advising against its use for people over 65 years. They consider the trials have not sufficiently demonstrated its efficiency for this group. So this could affect the authorisation by the European Commission of this vaccine for the elderly, who should be the first to be protected against the virus – 95% of the deaths resulting from Covid-19 are concentrated in the population over 70 years of age. In the medium term, the solution to the current problems of vaccine availability could be solved if other vaccines come into play, specifically those being developed by Johnson & Johnson and Novavax.
As far as Johnson & Johnson is concerned, early next week we could learn the results of the phase III trial of its Covid-19 vaccine, which began in Sept. 20. The most relevant aspect of its candidadcy is that a single dose would be enough to achieve protection and it is not necessary to maintain it at sub-zero temperatures. Meanwhile, Novavax has announced its high efficiency against the most widely spread variants of the virus (89% in its trial in the United Kingdom, 95% against the original strain and 85% against the British variant). However, its lower protection against new strains (49% in its trial in South Africa) is a cause for concern.
As far as the US is concerned, Biden has improved the vaccination target in his first 100 days in office from 100 million doses to 150 million doses. This means administering 1.5 million doses per day, 33% above the current rate of 1.1 million (seven-day average). They appear to be ambitious but achievable targets.
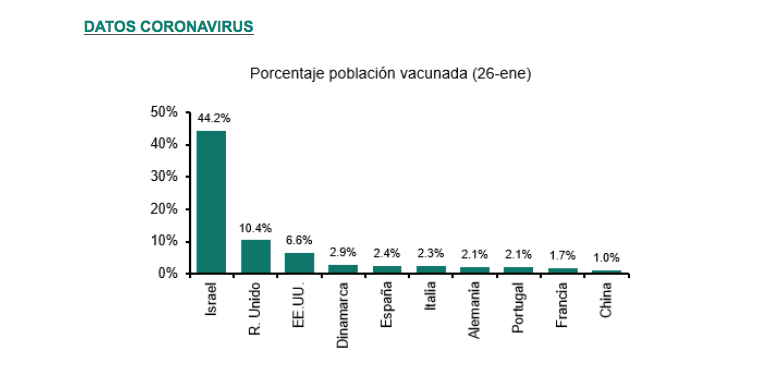
The following chart published a few days ago by Banca March shows the progress of the vaccination schedule in Europe and the rest of the world.